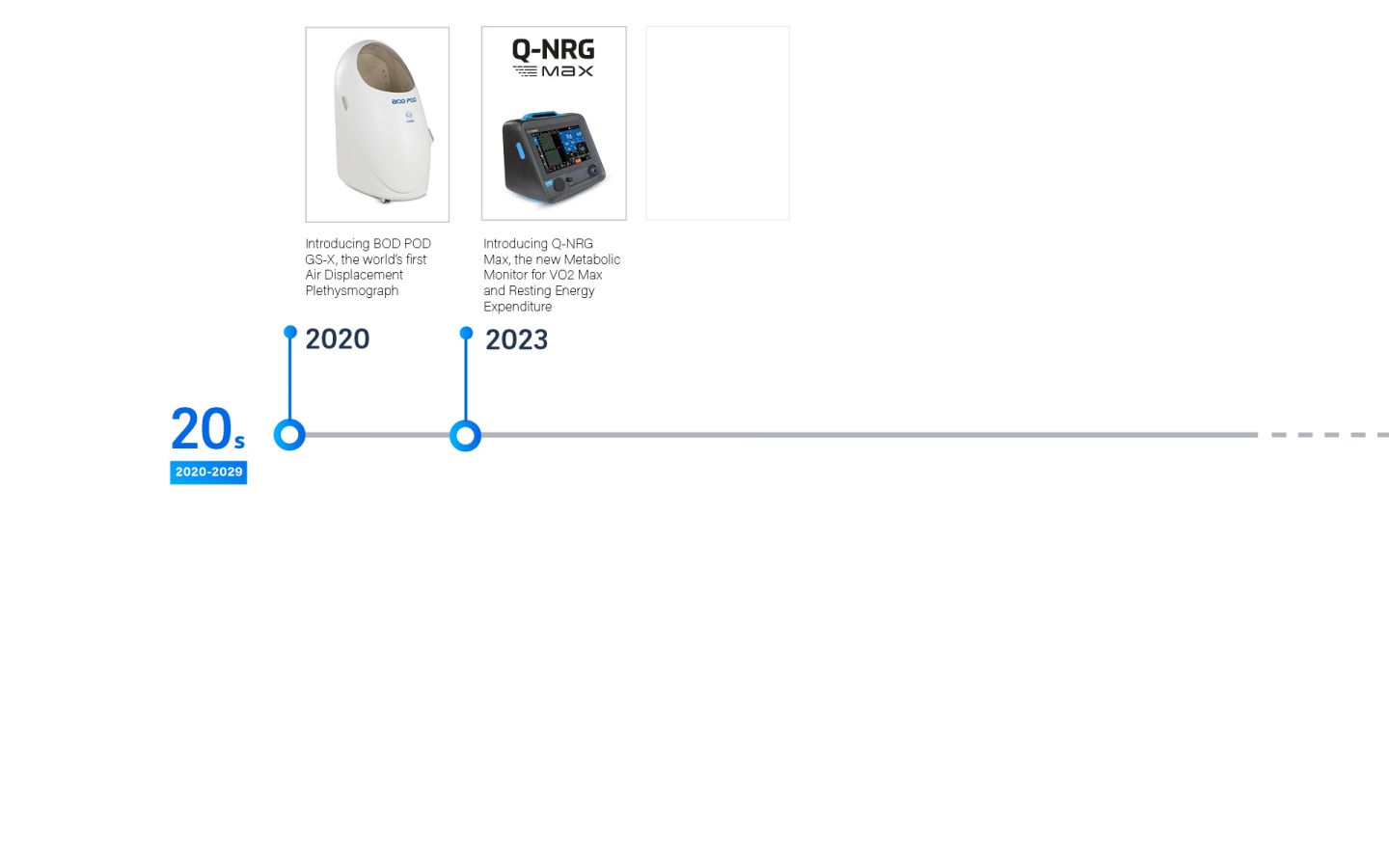
COSMED
Discover more about the Company, its history and key elements.
OVERVIEW
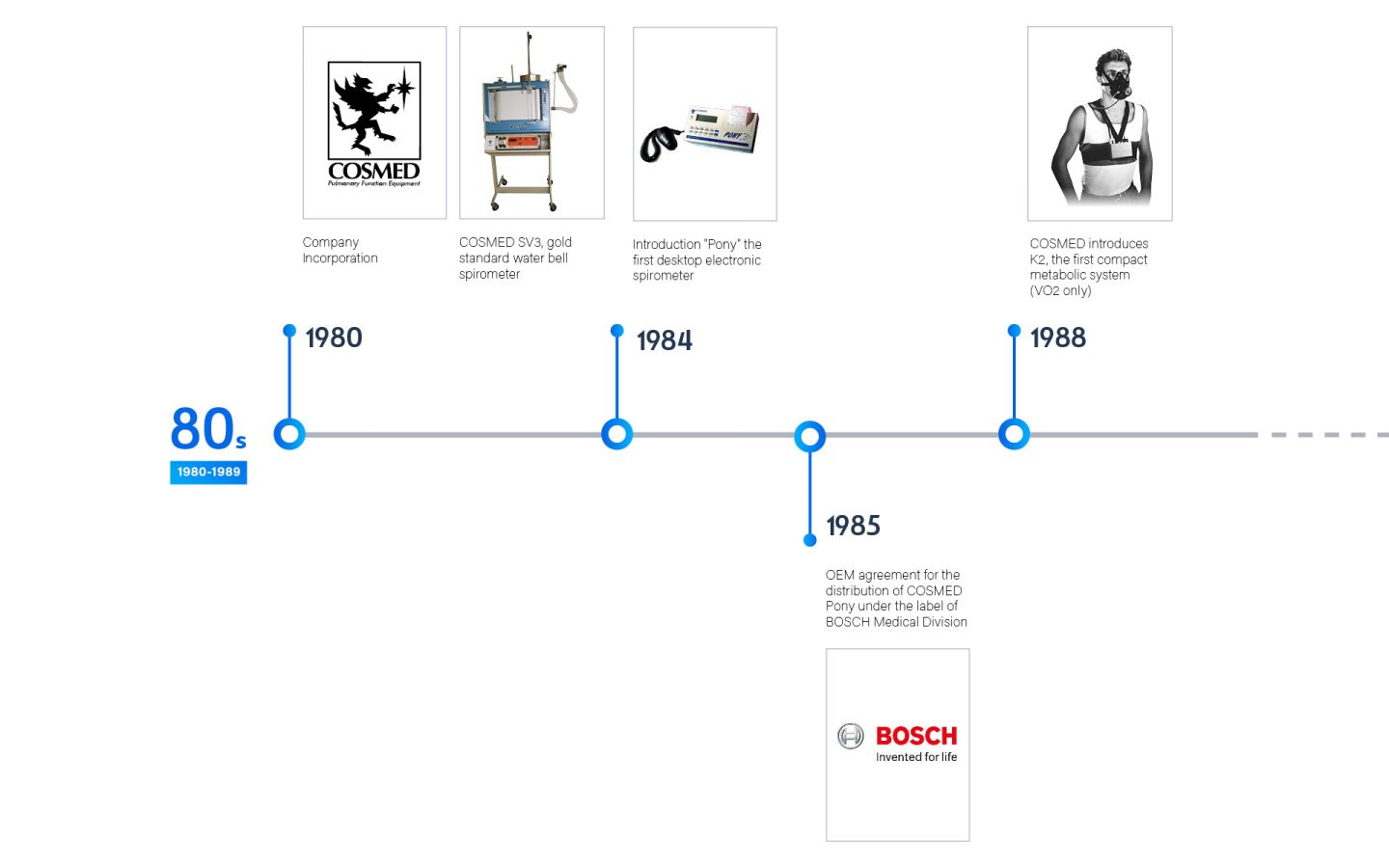
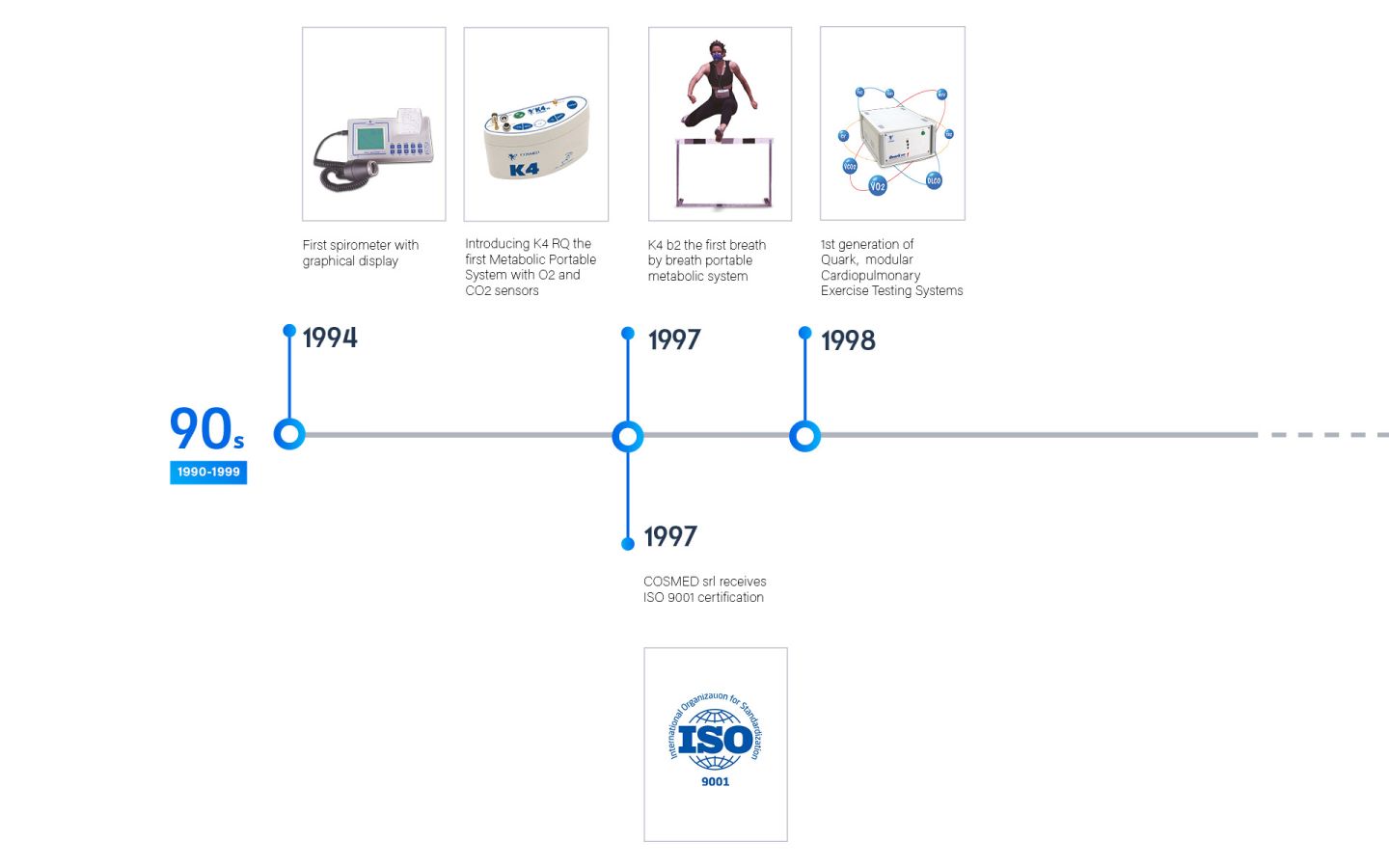
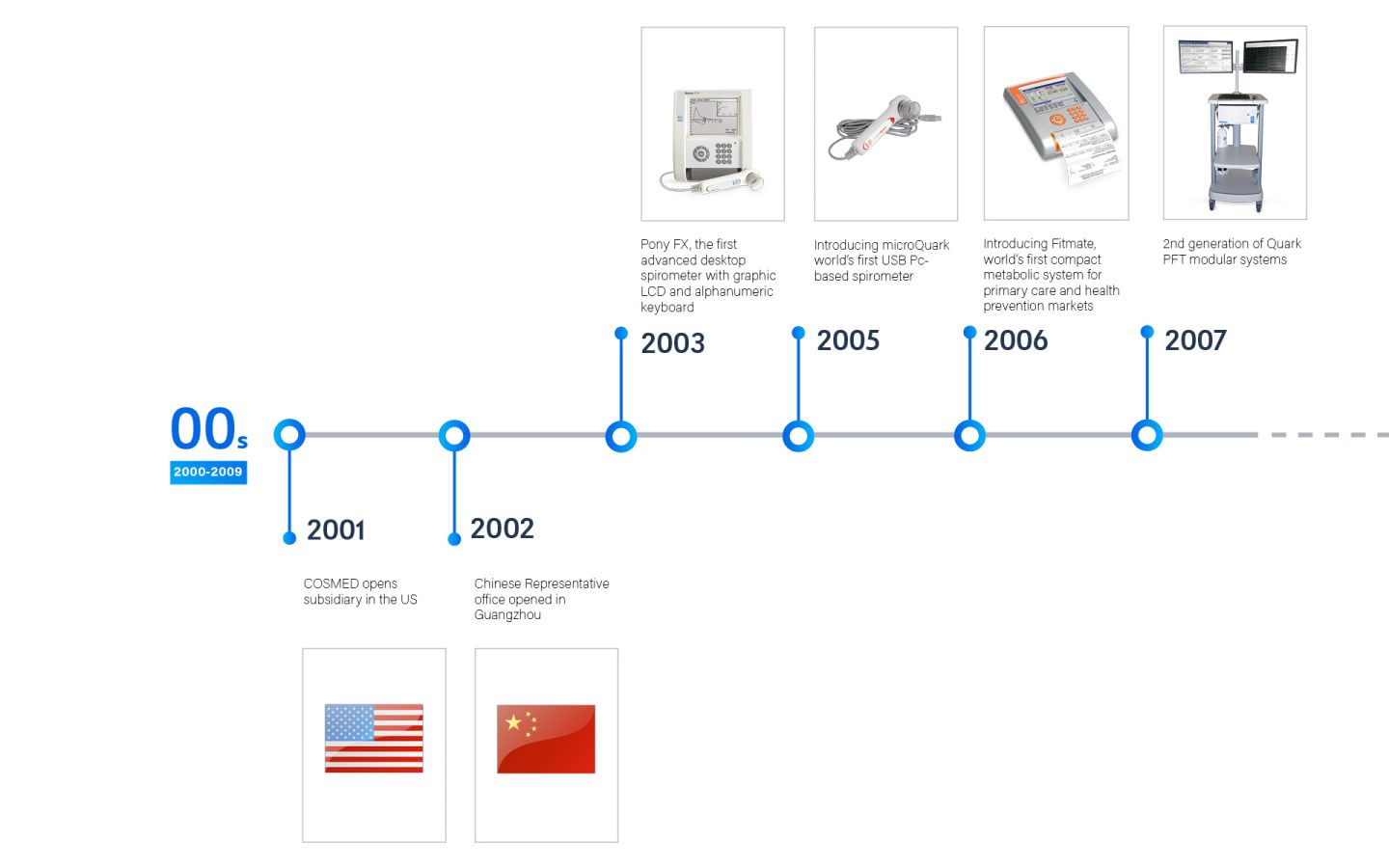
Founded in 1980, COSMED is an Italian company that specializes in the global design, manufacturing, and distribution of diagnostic medical devices. These devices serve a range of purposes, including the assessment of Lung function, Metabolic health, and Body Composition.
COSMED places great emphasis on maintaining the highest standards of quality and adheres to various quality system standards such as ISO 13485:2016, ISO 9001:2015, and MDSAP.
The medical devices developed by COSMED are meticulously designed to meet essential international and national product certifications, exemplified by the CE mark, FDA 510(k) clearance, and full compliance with major international safety standards like IEC 60601-1 (safety), IEC 60601-1-2 (electromagnetic compatibility), IEC 62304 (software), and IEC 62366 (usability). These certifications and standards ensure that COSMED's products are safe, effective, and reliable for use worldwide.
DOWNLOAD


KEY ELEMENTS
Corporate
Headquarters in Rome (Italy) with three manufacturing plants located in Italy, Denmark, and USA. COSMED has a global workforce of over 200 dedicated professionals.
Technology
COSMED is constantly committed to innovate and provide breakthrough technology in the field of cardio pulmonary diagnostics.
Intellectual Propriety
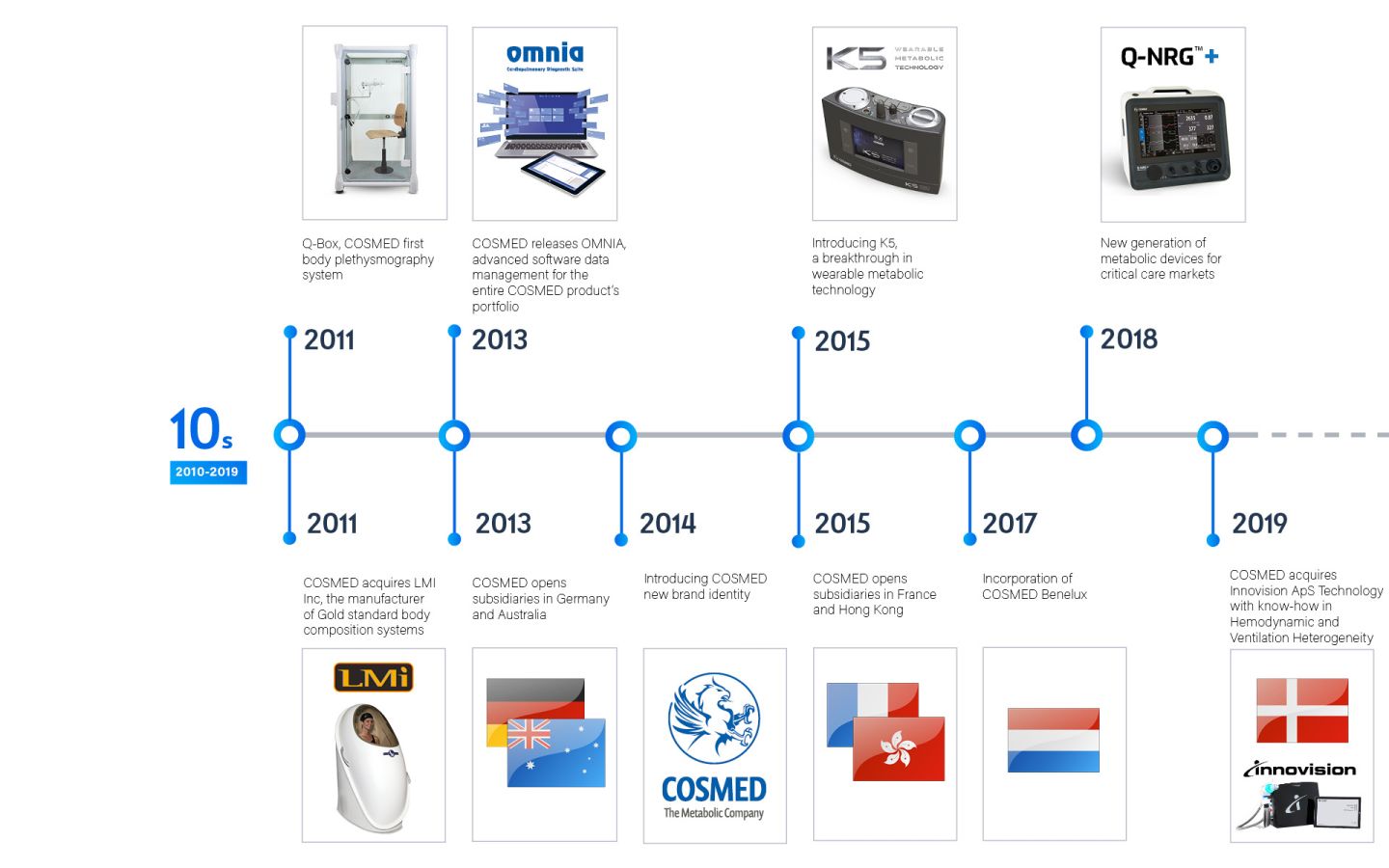
Throughout its existence, the company has made substantial investments in research and development, as well as human resources, leading to the creation of numerous international patents in Gas Exchange and Body Composition technology.
Scientific Community
Strong collaborations with International Scientific Communities and Academic and Research Organizations to support development and anticipate customer’s needs.
Global Network
COSMED presence is worldwide through its 8 subsidiaries and more than 60 Business Partners to cover more than 100 countries.
Customer Care
We pride ourselves on providing exceptional customer care on a global scale. Our services include first-class technical support, effective training programs, regular software updates, and the availability of genuine spare parts and consumables.

COSMED News
Read the latest news about our Company and discover the upcoming international Events
Get in touch
COSMED strives to provide the best service possible with every contact!
Fill the online forms to get the info you're looking for right now!